Should you have thoughts to the Company that issued the current document please Make contact with the company immediately.
(four) A listing of topics who dropped out through the study course from the investigation in Affiliation with any adverse practical experience, whether or not thought to be drug linked.
Why it’s superb: “Instagram is huge for models right this moment, so in-home social websites administrators and agency marketers require the most effective Resource they're able to rely on to provide their posts in quite possibly the most streamlined way doable.
A summary of preceding human encounter known towards the applicant, if any, with the investigational drug. The data is needed to include the next:
(one) A sponsor who wishes to demand for expanded entry to an investigational drug for treatment use underneath subpart I of this portion have to provide realistic assurance that charging will not interfere with producing the drug for marketing approval.
(iii) Reveal which the scientific demo could not be executed without charging because the cost of the drug is extraordinary into the sponsor. The fee may be incredible as a result of manufacturing complexity, scarcity of a normal useful resource, the big amount of drug needed (e.
(d) The IND structure established forth in § 312.23 should be adopted routinely by sponsors during the curiosity of fostering an successful critique of applications. Sponsors are envisioned to workout considerable discretion, however, concerning the content material of data submitted in Every area, depending on the kind of drug becoming examined and the character in the available facts. Area 312.23 outlines the information essential for your commercially sponsored IND for any new molecular entity. A sponsor-investigator who works by using, being a investigation Instrument, an investigational new drug that is certainly already subject matter to some producer's IND or marketing application should follow the very same common structure, but ordinarily may, if licensed through the maker, refer to the manufacturer's IND or marketing application in furnishing the technical info supporting the proposed medical investigation.
A quick assertion of another info that would assist analysis in the proposed clinical investigations with respect for their safety or their design and prospective as controlled medical trials to guidance marketing with the drug.
A transparent get in touch with-to-action – Your CTA button is intended to get them to choose the next stage. That may be signing up to your email record, scheduling a absolutely free phone, Or possibly even reserving an appointment. Social proof – This may be in the shape of recommendations or links back again to situation research on your website.
) An outline of scientific techniques, laboratory assessments, or other steps to get taken to watch the results of your drug in human topics and to attenuate danger.
The document summarizes the method for getting an Investigational New Drug Application (IND) from your FDA to carry out scientific trials of the experimental drug. It outlines exactly what is A part of an IND submission such as preclinical info, clinical protocols, manufacturing information and facts, and former human experience.
Investigational new drug application has to be submitted right after identifying a whole new drug and in advance of commencing of clinical trials. Right here specified a brief Be aware DeNovo on The subject.
The sponsor have to report any clinically vital rise in the speed of a serious suspected adverse reaction about that listed while in the protocol or investigator brochure.
(b) If an IND is withdrawn, FDA shall be so notified, all medical investigations performed under the IND shall be finished, all present-day investigators notified, and all stocks on the drug returned to the sponsor or in any other case disposed of for the request on the sponsor in accordance with § 312.fifty nine.
 Haley Joel Osment Then & Now!
Haley Joel Osment Then & Now! Brian Bonsall Then & Now!
Brian Bonsall Then & Now!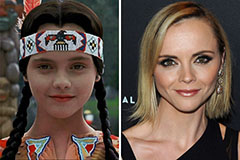 Christina Ricci Then & Now!
Christina Ricci Then & Now! Katey Sagal Then & Now!
Katey Sagal Then & Now! Naomi Grossman Then & Now!
Naomi Grossman Then & Now!